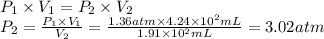Chemistry, 06.07.2020 17:01, lyssamichelle
A sample of oxygen, at 25°C, occupies a volume of 4.24 × 102 milliliters (mL) at 1.36 atm pressure. What pressure must be applied to compress the gas to a volume of 1.91 × 102 mL, with no temperature change?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 04:20, lelliott86
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 06:00, wirchakethan23
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 07:30, isalih7256
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1
Do you know the correct answer?
A sample of oxygen, at 25°C, occupies a volume of 4.24 × 102 milliliters (mL) at 1.36 atm pressure....
Questions in other subjects:


History, 10.12.2020 23:00

Mathematics, 10.12.2020 23:00


Spanish, 10.12.2020 23:00


English, 10.12.2020 23:00

Mathematics, 10.12.2020 23:00

Arts, 10.12.2020 23:00