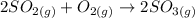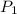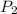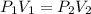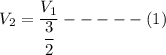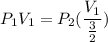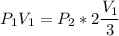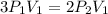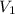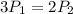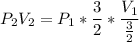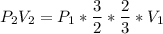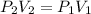Chemistry, 05.07.2020 14:01, jakobrobinette
Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) → 2SO3(g) Suppose the volume of this system is compressed to one-half its initial volume and then equilibrium is reestablished. The new equilibrium total pressure will be:

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3
Do you know the correct answer?
Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) → 2SO3(g) Suppose th...
Questions in other subjects:


Health, 21.04.2021 21:00





Mathematics, 21.04.2021 21:10


Mathematics, 21.04.2021 21:10

Mathematics, 21.04.2021 21:10