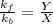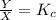Chemistry, 04.07.2020 14:01, reginaldboyd28
What is the relationship between the equilibrium constant (K c ) of reaction and the rate constants for the forward (k) and reverse (k r ) reactions in a single step reaction

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2


Chemistry, 23.06.2019 07:30, danielahumajova6
How do you interpret a chromagram for what mixtures contain?
Answers: 1
Do you know the correct answer?
What is the relationship between the equilibrium constant (K c ) of reaction and the rate constants...
Questions in other subjects:







Mathematics, 02.10.2019 08:30


English, 02.10.2019 08:30

Biology, 02.10.2019 08:30


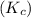 of reaction and the rate constants for the forward
of reaction and the rate constants for the forward 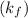 and reverse
and reverse 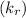 reactions in a single step reaction is
reactions in a single step reaction is 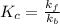
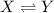
![K_c=\frac{[Y]}{[X]}](/tpl/images/0701/1901/3116c.png)
![k_f[X]](/tpl/images/0701/1901/d6b0b.png)
![k_b[Y]](/tpl/images/0701/1901/fc03b.png)