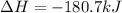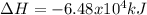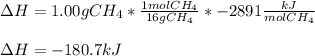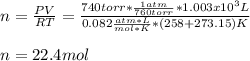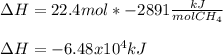Consider the following reaction:
CH4 +2O2 → CO2 + 2H2O. ΔH= -2891 kJ
Calculate the enth...

Chemistry, 04.07.2020 02:01, khikhi1705
Consider the following reaction:
CH4 +2O2 → CO2 + 2H2O. ΔH= -2891 kJ
Calculate the enthalpy change for each of the following cases:
a. 1.00 g methane is burned in excess oxygen.
b. 1.00 3 10^3 L methane gas at 740. torr and 258°C are burned in excess oxygen.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
Do you know the correct answer?
Questions in other subjects:


Mathematics, 10.12.2020 17:20

Biology, 10.12.2020 17:20

Engineering, 10.12.2020 17:20



Mathematics, 10.12.2020 17:20

English, 10.12.2020 17:20