Chemistry, 04.07.2020 01:01, Chandler1Gaming
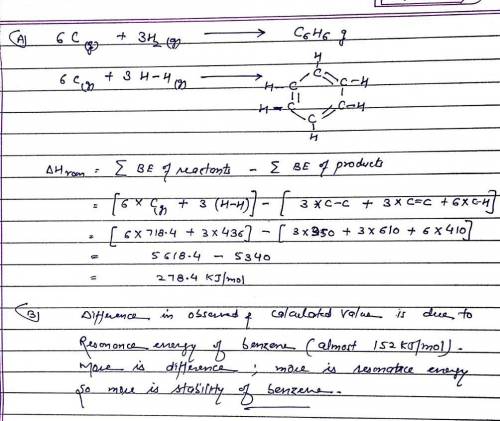
A. Use average bond energies together with the standard enthalpy of formation of C(g) (718.4 kJ/mol ) to estimate the standard enthalpy of formation of gaseous benzene, C6H6(g). (Remember that average bond energies apply to the gas phase only.) B. Compare the value you obtain using average bond energies to the actual standard enthalpy of formation of gaseous benzene, 82.9 kJ/mol. What does the difference between these two values tell you about the stability of benzene?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 00:00, juliannasl
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 08:00, IntellTanito
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
Do you know the correct answer?
A. Use average bond energies together with the standard enthalpy of formation of C(g) (718.4 kJ/mol...
Questions in other subjects:



Mathematics, 27.09.2020 14:01

Social Studies, 27.09.2020 14:01

Mathematics, 27.09.2020 14:01




Mathematics, 27.09.2020 14:01

Health, 27.09.2020 14:01