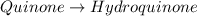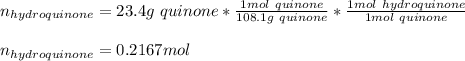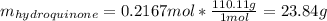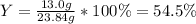Chemistry, 04.07.2020 01:01, allieb12334
Determine the theoretical maximum moles of hydroquinone, , that could be produced in this experiment. The reactant, quinone, is the limiting reagent. (To avoid introducing rounding errors on intermediate calculations, enter your answer to four significant figures.)
Reactant mass 23.4g
Product mass 13.0g
Reactant moles 0.2167 mol
Reactant mass 23.4g
Product mass 13.0g
Molar mass C 12.0 g/mol
Molar mass H 1.00 g/mol
Molar mass O 16.0 g/mol
Theoretical maximum moles of hydroquinone:

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Bradgarner772
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1

Do you know the correct answer?
Determine the theoretical maximum moles of hydroquinone, , that could be produced in this experiment...
Questions in other subjects:

English, 20.09.2019 00:30

Physics, 20.09.2019 00:30


Mathematics, 20.09.2019 00:30

Mathematics, 20.09.2019 00:30

Biology, 20.09.2019 00:30

Medicine, 20.09.2019 00:30