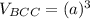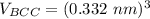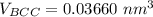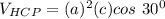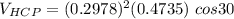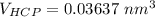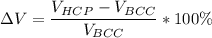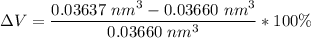Chemistry, 03.07.2020 23:01, harmonytaylor13
Above 882oC, zirconium has a BCC crystal structure with a = 0.332 nm. Below this temperature, zirconium has an HCP structure with a = 0.2978 nm and c = 0.4735 nm. Determine the percent volume change when BCC zirconium transforms to HCP zirconium. Is that contraction or expansion?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, 20heldmadison
Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make these assumptions: the combined weight of the pilot basket together with that of the balloon fabric and other equipment is w. the volume of the hot air inside the balloon when it is inflated is v. the absolute temperature of the hot air at the bottom of the balloon is th (where th> tc). the absolute temperature of the cold air outside the balloon is tc and its density is ďc. the balloon is open at the bottom, so that the pressure inside and outside the balloon is the same. as always, treat air as an ideal gas. use g for the magnitude of the acceleration due to gravity.
Answers: 1

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2
Do you know the correct answer?
Above 882oC, zirconium has a BCC crystal structure with a = 0.332 nm. Below this temperature, zircon...
Questions in other subjects:





Mathematics, 29.10.2020 02:00


Mathematics, 29.10.2020 02:00

Mathematics, 29.10.2020 02:00