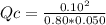The reaction of nitrogen gas and oxygen gas to form nitrogen monoxide gas is shown below. If the measured concentrations of all three chemicals at some point in time are: [N2] = 0.80 M
[O2] = 0.050 M
[NO] = 0.10 M
Which statement is TRUE about the reaction at this point in time? N2(g) + O2(g) ⇄ 2 NO(g) K = 0.10
The reaction is at equilibrium.
The reverse reaction is occurring at a faster rate than the forward reaction.
The forward reaction is occurring at a faster rate than the reverse reaction.
This set of concentration values is impossible because the concentrations of N2 and O2 must be the same.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

Chemistry, 23.06.2019 00:00, maronetham6253
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 03:20, elijahmoore841
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
Do you know the correct answer?
The reaction of nitrogen gas and oxygen gas to form nitrogen monoxide gas is shown below. If the mea...
Questions in other subjects:

Mathematics, 17.07.2019 13:00

English, 17.07.2019 13:00

Geography, 17.07.2019 13:00


Geography, 17.07.2019 13:00

Biology, 17.07.2019 13:00

Chemistry, 17.07.2019 13:00


Biology, 17.07.2019 13:00

Biology, 17.07.2019 13:00


![Qc=\frac{[C]^{c}*[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0700/8667/038b9.png)
![Qc=\frac{[NO]^{2} }{[N_{2} ]*[O_{2} ] }](/tpl/images/0700/8667/47464.png)