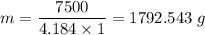Chemistry, 03.07.2020 17:01, Wanna14ever
What mass of water uses 7500 J to increase the temperature from 2 oC to 3 oC? The following information for water is given, but may or may not be useful: Δc = 4.184 J/goC ΔHfus = 334 J/g ΔHvap= 2260J/g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 01:00, skatelife8974
What is the chemical name of the compound ti2o3?
Answers: 2


Chemistry, 23.06.2019 07:30, 22emilyl530
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
Do you know the correct answer?
What mass of water uses 7500 J to increase the temperature from 2 oC to 3 oC? The following informat...
Questions in other subjects:

Mathematics, 01.09.2019 09:50


English, 01.09.2019 09:50


History, 01.09.2019 09:50





Mathematics, 01.09.2019 09:50


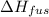 = 334 J/g
= 334 J/g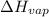 = 2260 J/g
= 2260 J/g