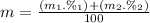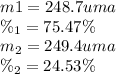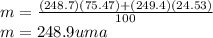Chemistry, 02.10.2019 14:50, Jsquad8879
Calculate the average atomic mas of an unknown element that has two naturally-occurring isotopes.
the first isotope occurs 75.47% of the time and has a mass of 248.7 a. m.u.
the second isotope occurs 24.53% of the time and has a mass of 249.4 a. m.u.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3


Chemistry, 23.06.2019 00:00, ahmedeldyame
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
Do you know the correct answer?
Calculate the average atomic mas of an unknown element that has two naturally-occurring isotopes.
Questions in other subjects:

Health, 30.06.2019 01:30



Mathematics, 30.06.2019 01:30

History, 30.06.2019 01:30


Mathematics, 30.06.2019 01:30


Arts, 30.06.2019 01:30

English, 30.06.2019 01:30