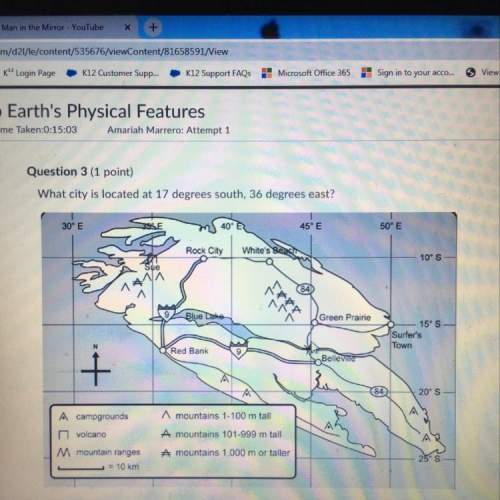
Chemistry, 01.07.2020 23:01, Hjackson24
HELP ASAP: Determine the enthalpy change of the following reaction: CH4 + 4Cl2 -> CCl4+ 4HCl
Given enthalpies:
CH4: -17.9 kJ/mol
Cl2: 0 kJ/mol
CCl4: -95.98 kJ/mol
HCl: -92.3 kJ/mol
A. -447.28 kJ/mol
B. -206.18
C. -170.38
D. -393.58

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 23.06.2019 01:00, kwarwick0915
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 04:31, CassidgTab
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3
Do you know the correct answer?
HELP ASAP: Determine the enthalpy change of the following reaction: CH4 + 4Cl2 -> CCl4+ 4HCl
Gi...
Questions in other subjects:

English, 26.09.2019 03:40



English, 26.09.2019 03:40

English, 26.09.2019 03:40

English, 26.09.2019 03:40

English, 26.09.2019 03:40


English, 26.09.2019 03:40

English, 26.09.2019 03:40