
Chemistry, 01.07.2020 16:01, savannahsims5847
The iodine clock experiment consists of the following three reactions, where Reaction 1 is slow relative to Reactions 2 and 3. Predict what would happen if Reaction 2 was slow relative to Reaction 1
Reaction 1:3I^- (aq) + S_2O_8^2- (aq) rightarrow I_3^-, (aq) + 2SO_4^2- (aq) slow
Reaction 2: l_3^-(aq) + 2S_2O_3^2- (aq) rightarrow 3I^- (aq) + S_4O_6^2- (aq) fast
Reaction 3: l_3^- (aq) + starch (aq) rightarrow 3I^ starch (bluish black) fast
A. A color change would appear immediately because I_3^+, would be removed slowly from solution, and would therefore be able to react with starch.
B. The reaction would turn blue slower because I_3^-, is removed at a slower rate.
C. The rate of color change would indicate the rate of reaction 3 instead of reaction 1
D. Reaction 3 would not occur.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, ayoismeisalex
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
Do you know the correct answer?
The iodine clock experiment consists of the following three reactions, where Reaction 1 is slow rela...
Questions in other subjects:


English, 13.01.2020 20:31


Biology, 13.01.2020 20:31

Law, 13.01.2020 20:31

Physics, 13.01.2020 20:31

Business, 13.01.2020 20:31


Mathematics, 13.01.2020 20:31

Social Studies, 13.01.2020 20:31


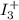 , would be removed slowly from solution, and would therefore be able to react with starch, is the correct answer.
, would be removed slowly from solution, and would therefore be able to react with starch, is the correct answer.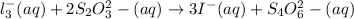
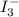 will be slow in nature.
will be slow in nature.




