
Consider the general reversible reaction. Lower A upper A plus lower B upper B double-headed arrow lower C upper C plus Lower d upper D. What is the equilibrium constant expression for the given system? K e q equals StartFraction lowercase C StartBracket upper C EndBracket lowercase d StartBracket upper D EndBracket over lowercase A StartBracket upper A EndBracket lowercase B StartBracket upper B EndBracket EndFraction. K e q equals StartFraction StartBracket upper C EndBracket StartBracket upper D EndBracket over StartBracket upper A EndBracket StartBracket upper B EndBracket EndFraction. K e q equals StartFraction StartBracket upper A EndBracket superscript lower a StartBracket upper B EndBracket superscript lower b over StartBracket upper C EndBracket superscript lower c StartBracket upper D EndBracket superscript lower D EndFraction. K e q equals StartFraction StartBracket upper C EndBracket superscript lower c StartBracket upper D EndBracket superscript lower D over StartBracket upper A EndBracket superscript lower a StartBracket upper B EndBracket superscript lower b EndFraction.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 18:00, liddopiink1
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3
Do you know the correct answer?
Consider the general reversible reaction. Lower A upper A plus lower B upper B double-headed arrow l...
Questions in other subjects:




History, 28.07.2019 14:00

Social Studies, 28.07.2019 14:00

Mathematics, 28.07.2019 14:00

History, 28.07.2019 14:00

English, 28.07.2019 14:00

History, 28.07.2019 14:00

Mathematics, 28.07.2019 14:00


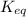
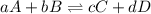
![K_{eq}=\frac{[C]^c\times [D]^d}{[A]^a\times [B]^b}](/tpl/images/0696/7940/ed1ff.png)




