
Chemistry, 28.06.2020 15:01, Destinyb3722
Will GIVE BRAINLIEST --A student makes a standard solution of potassium hydroxide by adding 14.555 g to 500.0 mL of water. Answer the following questions, being sure to include units and remember sig figs. Show your work. a. What is the concentration of this standard solution?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 05:00, rosezgomez97
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Chemistry, 23.06.2019 10:20, NasirKA7372
What do many bases have in common? apex chemistry sem 2
Answers: 2


Chemistry, 23.06.2019 21:30, saintsfan2004
Which class of compunds has the general formula r-o-r
Answers: 1
Do you know the correct answer?
Will GIVE BRAINLIEST --A student makes a standard solution of potassium hydroxide by adding 14.555 g...
Questions in other subjects:

Mathematics, 22.05.2021 20:20

Mathematics, 22.05.2021 20:20

Mathematics, 22.05.2021 20:20

History, 22.05.2021 20:20

Chemistry, 22.05.2021 20:20


Mathematics, 22.05.2021 20:20

English, 22.05.2021 20:30



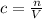
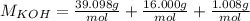

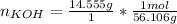

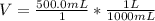
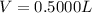
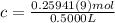
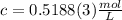 <= Keep an insignificant figure for rounding
<= Keep an insignificant figure for rounding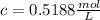 <= Rounded up
<= Rounded up <= You use the unit "M" instead of "mol/L"
<= You use the unit "M" instead of "mol/L"




