Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, celestemaria0727
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3
Do you know the correct answer?
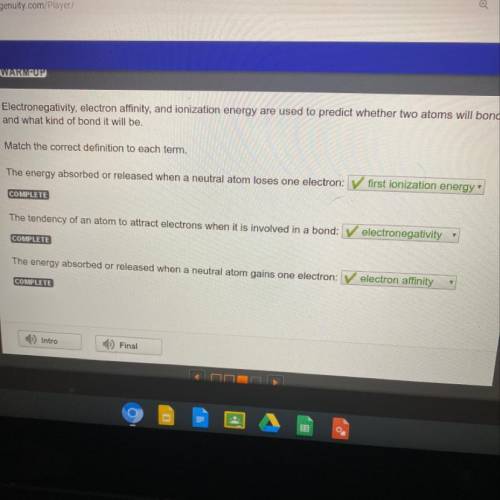
Electronegativity, electron
affinity, and ionization energy are used to predict whether two atoms w...
Questions in other subjects:


Biology, 29.10.2021 05:40


Mathematics, 29.10.2021 05:40

Business, 29.10.2021 05:40


Mathematics, 29.10.2021 05:40

Mathematics, 29.10.2021 05:40


Mathematics, 29.10.2021 05:40