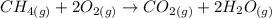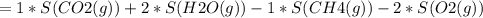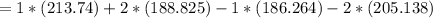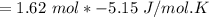Chemistry, 27.06.2020 18:01, destiny3200
Consider the reaction CH4(g) 2O2(g)CO2(g) 2H2O(g) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 1.62 moles of CH4(g) react at standard conditions.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
Do you know the correct answer?
Consider the reaction CH4(g) 2O2(g)CO2(g) 2H2O(g) Using standard thermodynamic data at 298K, calcula...
Questions in other subjects:

Health, 04.03.2021 03:00




Mathematics, 04.03.2021 03:00

Engineering, 04.03.2021 03:00

Mathematics, 04.03.2021 03:00


Mathematics, 04.03.2021 03:00