
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and in a coffee-cup calorimeter. In one measurement, qrxn=−13.3qrxn=−13.3 kJkJ in the other qrxn=−11.9qrxn=−11.9 kJkJ. Which value was obtained in the bomb calorimeter? (Assume that the reaction has a positive ΔVΔV in the coffee-cup calorimeter.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2



Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
Do you know the correct answer?
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and...
Questions in other subjects:











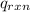 = -13.3 KJ belongs to the bomb calorimeter
= -13.3 KJ belongs to the bomb calorimeter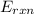 of the reaction, the work of the reaction
of the reaction, the work of the reaction 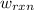 and the heat of the reaction
and the heat of the reaction 



