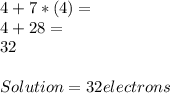calculating A, the available


Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 23.06.2019 00:30, Keemdadream13
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
Do you know the correct answer?
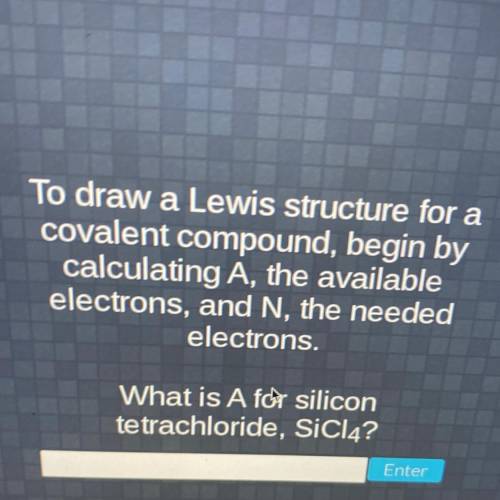
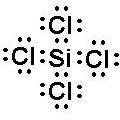
To draw a Lewis structure for a
covalent compound, begin by
calculating A, the available
calculating A, the available
Questions in other subjects:

Chemistry, 12.12.2020 16:50





Mathematics, 12.12.2020 16:50


Spanish, 12.12.2020 16:50

Health, 12.12.2020 16:50

Computers and Technology, 12.12.2020 16:50