
Chemistry, 27.06.2020 02:01, sairaanwar67
Consider the following reversible reaction. CO(g)+2H2(g) CH3OH(g) What is the equilibrium constant expression for the given system?
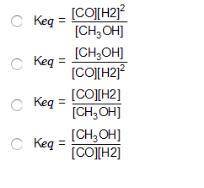
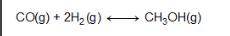

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3


Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
Do you know the correct answer?
Consider the following reversible reaction. CO(g)+2H2(g) CH3OH(g) What is the equilibrium constant e...
Questions in other subjects:


Biology, 17.03.2021 23:40


Mathematics, 17.03.2021 23:40




Mathematics, 17.03.2021 23:40


History, 17.03.2021 23:40






