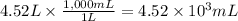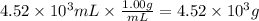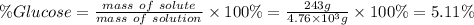Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Do you know the correct answer?
What is the mass percent of glucose solute of a solution that is composed of 243g of glucose and 4.5...
Questions in other subjects:

Mathematics, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01


Mathematics, 06.05.2020 15:01

English, 06.05.2020 15:01



Biology, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01