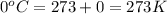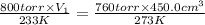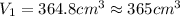Chemistry, 26.06.2020 16:01, normarjohnson
A certain volume of a gas had a pressure of 800 torr at a temperature of -40 degrees C. What was the original volume if the volume at STP is now 450.0 cm^3? (the correct answer is 365 cm^3. I just need an explanation.)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, timiaparker
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 07:00, shradhwaip2426
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 21:30, steven0448
An atomic nucleus is composed ofa)protons. b)protons and neutrons. c)protons and electrons. d)protons, neutrons, and electrons.
Answers: 1
Do you know the correct answer?
A certain volume of a gas had a pressure of 800 torr at a temperature of -40 degrees C. What was the...
Questions in other subjects:

Mathematics, 10.02.2021 23:30

History, 10.02.2021 23:30

Physics, 10.02.2021 23:30

English, 10.02.2021 23:30


Mathematics, 10.02.2021 23:30


Mathematics, 10.02.2021 23:30


History, 10.02.2021 23:30


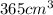
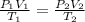
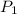 = initial pressure of gas = 800 torr
= initial pressure of gas = 800 torr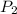 = final pressure of gas at STP = 760 torr
= final pressure of gas at STP = 760 torr
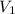 = initial volume of gas = ?
= initial volume of gas = ?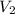 = final volume of gas at STP =
= final volume of gas at STP = 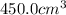
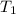 = initial temperature of gas =
= initial temperature of gas = 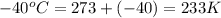
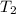 = final temperature of gas at STP =
= final temperature of gas at STP =