
Chemistry, 26.06.2020 16:01, AnxiousKid
An equilibrium mixture of the three gases in a 1.00 L flask at 350 K contains 5.35×10-2 M CH2Cl2, 0.173 M CH4 and 0.173 M CCl4. What will be the concentrations of the three gases once equilibrium has been reestablished, if 0.155 mol of CH4(g) is added to the flask?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:30, lizdeleon248
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 00:00, PineappleDevil889
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1


Chemistry, 23.06.2019 08:00, IntellTanito
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
Do you know the correct answer?
An equilibrium mixture of the three gases in a 1.00 L flask at 350 K contains 5.35×10-2 M CH2Cl2, 0....
Questions in other subjects:


English, 24.03.2021 17:10




Chemistry, 24.03.2021 17:10



Spanish, 24.03.2021 17:10

Physics, 24.03.2021 17:10


![K = \frac{[CH_{4}][CCl_{4}]}{[CH_{2}Cl_{2}]^{2}} = \frac{0.173 M*0.173 M}{(5.35 \cdot 10^{-2} M)^{2}} = 10.5](/tpl/images/0695/0052/aba2c.png)

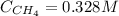
![K = \frac{[CH_{4}][CCl_{4}]}{[CH_{2}Cl_{2}]^{2}} = \frac{(0.328 + x)(0.173 + x)}{(5.35 \cdot 10^{-2} - 2x)^{2}}](/tpl/images/0695/0052/cf9a0.png)





