
Chemistry, 25.06.2020 02:01, brookcoyanpeus5y
The acetate ion is the conjugate base of the weak acid acetic acid. The value of Kb for CH3COO-, is 5.56×10-10. Write the equation for the reaction that goes with this equilibrium constant.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
Do you know the correct answer?
The acetate ion is the conjugate base of the weak acid acetic acid. The value of Kb for CH3COO-, is...
Questions in other subjects:


English, 21.01.2021 21:40


Biology, 21.01.2021 21:40

World Languages, 21.01.2021 21:40

English, 21.01.2021 21:40

Mathematics, 21.01.2021 21:40

Mathematics, 21.01.2021 21:40



![5.56\times 10^{-10}=\frac{[CH_3COOH]}{[CH_3COO^-]\times [H^+]}](/tpl/images/0693/5990/d4718.png)

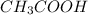 donates a proton and thus behaves as an acid and forms
donates a proton and thus behaves as an acid and forms 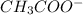 which is called as the conjugate base of
which is called as the conjugate base of 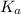 and the dissociation constant of bases is given by the term
and the dissociation constant of bases is given by the term  and is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios.
and is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios.![K_a=\frac{[CH_3COO^-]\times [H^+]}{[CH_3COOH]}](/tpl/images/0693/5990/0c492.png)

![K_b=\frac{[CH_3COOH]}{[CH_3COO^-]\times [H^+]}](/tpl/images/0693/5990/71be5.png)





