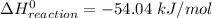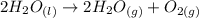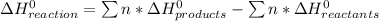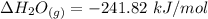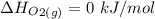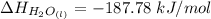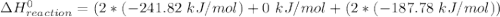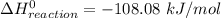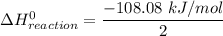The decomposition of hydrogen peroxide, H2O2, has been used to provide thrust in the control jets of various space vehicles. Using the data in Appendix G, determine how much heat is produced by the decomposition of exactly 1 mole of H2O2 under standard conditions. 2H2 O2 (l) ⟶ 2H2 O(g) + O2 (g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, thebrain1345
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2


Chemistry, 22.06.2019 22:30, jaylenmiller437
The diagram shows the relationship between scientific disciplines. the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a. physics b. biology c. chemistry d. metallurgy
Answers: 2

Chemistry, 22.06.2019 23:30, lizdeleon248
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
Do you know the correct answer?
The decomposition of hydrogen peroxide, H2O2, has been used to provide thrust in the control jets of...
Questions in other subjects:

Mathematics, 26.05.2021 17:10

Mathematics, 26.05.2021 17:10