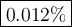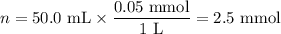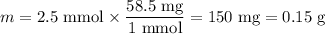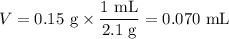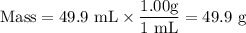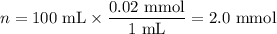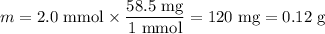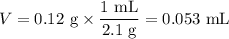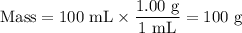Chemistry, 24.06.2020 06:01, maevemboucher78
8. A 50.0 mL 0.05 mol/l solution of sodium cloride (NaCl) was mixed with 100.0 mL
of 0.02 mol/l NaCl solution. What is the mass percent of NaCl in the final solution?
Assume the volumes are additive and their densities 21 g/mL. The molar mass of
NaCl is 58.5 g/mol. (10 points)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:30, koggebless
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

Chemistry, 23.06.2019 11:40, heyheyheyhey3
Which of the following is true for a reliable scientific source? it cites logic. it cites opinions. it cites valid data. it cites common sense.
Answers: 2
Do you know the correct answer?
8. A 50.0 mL 0.05 mol/l solution of sodium cloride (NaCl) was mixed with 100.0 mL
of 0.02 mol/l NaC...
Questions in other subjects:

Biology, 23.03.2021 21:20

English, 23.03.2021 21:20


Mathematics, 23.03.2021 21:20



Mathematics, 23.03.2021 21:20

History, 23.03.2021 21:20