Chemistry, 23.06.2020 17:01, jluckie080117
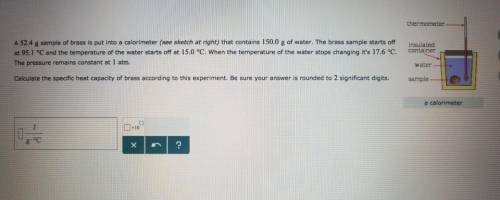
A sample of brass is put into a calorimeter (see sketch at right) that contains of water. The brass sample starts off at and the temperature of the water starts off at . When the temperature of the water stops changing it's . The pressure remains constant at . Calculate the specific heat capacity of brass according to this experiment. Be sure your answer is rounded to significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, lindseyklewis1p56uvi
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1


Chemistry, 22.06.2019 12:00, sophiaa23
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
Do you know the correct answer?
A sample of brass is put into a calorimeter (see sketch at right) that contains of water. The brass...
Questions in other subjects:

History, 22.10.2019 15:50





Mathematics, 22.10.2019 15:50

History, 22.10.2019 15:50




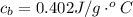
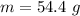
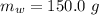
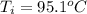
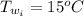
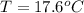
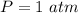
![H_L = m * c_b * [T_i - T]](/tpl/images/0692/3585/a3617.png)
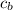 is the specific neat of the brass sample
is the specific neat of the brass sample ![H_g = m_w *c_w * [T_w - T ]](/tpl/images/0692/3585/35bc1.png)
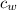 is the specific heat of water which has a constant value of
is the specific heat of water which has a constant value of 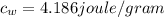
![H_L = H_g \ \equiv m* c_b * [T_i -T] = m_w * c_w * [T - T_w]](/tpl/images/0692/3585/4fcd7.png)
![52.4 * c_b * [95.1 - 17.6] = 150 * 4.186 * [ 17.6 - 15.0]](/tpl/images/0692/3585/31561.png)