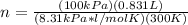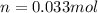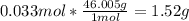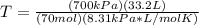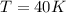PLEASE HELP CHEM BABES I HAVE BEEN CRYING FOR A WHILE NOW
1. Calculate the mass of nitrogen dioxide (NO2) present in a 0.831 L container if the pressure is 100 kPa at a temperature of 27 oC. R = 8.31 kPa x L / mol x K. (K = oC + 273).
2. A 33.2 L tank contains 280 g of compressed helium. If the pressure inside the tank is 700.0 kPa, what is the temperature of the compressed gas? You must convert the mass of helium into moles using the molar mass of He. The conversion factor will be 1 mol / molar mass of helium. R = 8.31 kPa x L / mol x K

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 03:30, tamariarodrigiez
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
Do you know the correct answer?
PLEASE HELP CHEM BABES I HAVE BEEN CRYING FOR A WHILE NOW
1. Calculate the mass of nitrogen dioxide...
Questions in other subjects:


Biology, 05.11.2020 05:40

Mathematics, 05.11.2020 05:40





English, 05.11.2020 05:40


Mathematics, 05.11.2020 05:40