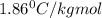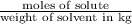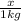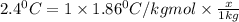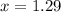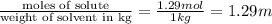Chemistry, 22.06.2020 00:57, Tianylee2328
How many moles of ethylene glycol must be added to 1 kg of water to make a solution with a freezing point of -2.4°C? The freezing point depression constant for water is 1.86°C•kg/mol. What is the molality of the solution?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 05:00, pmbeachy3102
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2

Chemistry, 23.06.2019 10:00, alejandra216
You dissolve 8.65 grams of lead(l) nitrate in water and then you add 2 50 grams of aluminum. this reaction occurs 2ai(s)+ 3pb(no3)2(aq) -3pb(s)+ 2aino3la(aq) the theoretical yield of solid lead?
Answers: 1
Do you know the correct answer?
How many moles of ethylene glycol must be added to 1 kg of water to make a solution with a freezing...
Questions in other subjects:


Biology, 29.10.2020 21:20

Social Studies, 29.10.2020 21:20






Mathematics, 29.10.2020 21:20


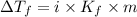
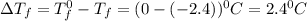 = Depression in freezing point
= Depression in freezing point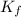 = freezing point constant for water=
= freezing point constant for water=