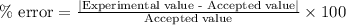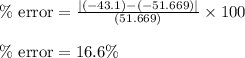Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 22:30, needhelpasap8957
Why is the bottom layer of a trophic pyrimid the
Answers: 2

Chemistry, 22.06.2019 22:30, creepycrepes
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
Do you know the correct answer?
If the theoretical value for AH of the reaction HCl + NH 3 NH 4 Cl is -51.669 kJ/mol, but from your...
Questions in other subjects:

Social Studies, 25.06.2019 12:00



Advanced Placement (AP), 25.06.2019 12:00




History, 25.06.2019 12:00