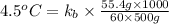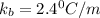Chemistry, 21.06.2020 06:57, ethanmatthews2336
A certain substance X condenses at a temperature of 120.7 degree C. But if a 500, g sample of X is prepared with 55.4 g of urea (NH_2)_2 CO) dissolved in it, the sample is found to have a condensation point of 125.2 degree C instead. Calculate the molal boiling point elevation constant K_b of X. Round your answer to 2 significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1

Chemistry, 23.06.2019 04:00, onegirl435
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1

Do you know the correct answer?
A certain substance X condenses at a temperature of 120.7 degree C. But if a 500, g sample of X is p...
Questions in other subjects:



Mathematics, 17.02.2021 01:40





Social Studies, 17.02.2021 01:40

Social Studies, 17.02.2021 01:40

Physics, 17.02.2021 01:40


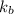 of X is
of X is 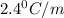
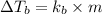
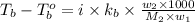
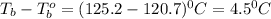
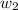 = mass of solute (urea) = 55.4 g
= mass of solute (urea) = 55.4 g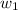 = mass of solvent X = 500 g
= mass of solvent X = 500 g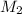 = molar mass of solute (urea) = 60 g/mol
= molar mass of solute (urea) = 60 g/mol