
Chemistry, 27.09.2019 06:30, niyyyareligion
If 5 moles of p4 reacted with 22 moles cl2 according to the above reaction, determine:
a) how many moles pcl3 are produced
b) how many moles of p4 are left in excess after the reaction (if any)
c) how many moles of cl2 are left in excess after the reaction (if any)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2



Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
Do you know the correct answer?
If 5 moles of p4 reacted with 22 moles cl2 according to the above reaction, determine:
a) how...
a) how...
Questions in other subjects:


Mathematics, 09.10.2019 06:30

Mathematics, 09.10.2019 06:30



Social Studies, 09.10.2019 06:30

History, 09.10.2019 06:30

Mathematics, 09.10.2019 06:30



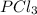 = 2.44 moles
= 2.44 moles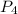 left = 1.34 mole
left = 1.34 mole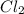 because it is completely consumed in the reaction.
because it is completely consumed in the reaction.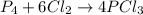
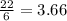 moles of
moles of 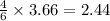 moles of
moles of 



