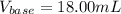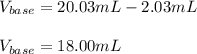Chemistry, 20.06.2020 17:57, seaotter7140
uppose you are titrating an acid of unknown concentration with a standardized base. At the beginning of the titration, you read the base titrant volume as 2.03 mL. After running the titration and reaching the endpoint, you read the base titrant volume as 20.03 mL. What volume of base was required for the titration

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 03:20, coollid876
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 07:40, Aaron5795
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 10:40, Dvrsug8598
Aliquid solution can be made select all that apply. dissolving solids into liquids, mixing liquids, dissolving gas solutes into liquids , mixing gases, mixing solids
Answers: 3

Chemistry, 23.06.2019 15:30, live4dramaoy0yf9
Amole, which is a unit in chemistry, contains 6.02 x 1023 atoms or particles. how many zeroes follow the 2 when this number is written out in standard form? a) 21 b) 23 c) 24 d) 25
Answers: 1
Do you know the correct answer?
uppose you are titrating an acid of unknown concentration with a standardized base. At the beginning...
Questions in other subjects:

Business, 05.10.2021 19:10



Mathematics, 05.10.2021 19:10

History, 05.10.2021 19:10