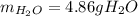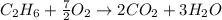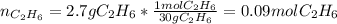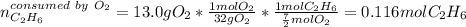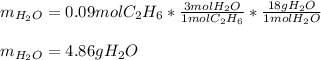Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 2.7 g of ethane is mixed with 13.0 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, drivinghydra
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2
Do you know the correct answer?
Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions in other subjects:




Mathematics, 24.03.2020 03:27




Mathematics, 24.03.2020 03:27

Mathematics, 24.03.2020 03:27