
Chemistry, 20.06.2020 12:57, mustafaserang582
2. Levetiracetam is marketed under the trade name Keppra it is a drug used for to prevent seizures. The
chemical formula for this drug is C&H14N202.
a. What is the molar mass of this drug? 170.209 g/mol
b. How many moles are in 15mg of this drug (1000mg = 1 gram)?
c. How many molecules are in 15mg of this drug?
d. How many oxygen atoms are in 15mg of this drug?
e. How many aitrogen atoms are in 15mg of this drug?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3



Do you know the correct answer?
2. Levetiracetam is marketed under the trade name Keppra it is a drug used for to prevent seizures....
Questions in other subjects:


Chemistry, 25.06.2021 03:00









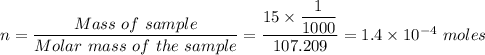
 × n
× n



