
Chemistry, 20.06.2020 08:57, graciewyatt471
1) How many kJ are absorbed when 45.2 g of water at 31.3 oC is heated to 76.9 oC? 2) Calculate the total heat released in kcal when 72.1 g water at 25.2 oC is cooled to 0 oC and freezes. 3) How many kilojoules are required to heat 55,500 mg of gold with specific heat = 0.129 J/g oC is heated from 24.6 oC to 123.4 oC? 4) Calculate the heat needed in kcal to change 45.6 g of water at 100 oC to change into steam.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, DarcieMATHlin2589
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 23.06.2019 10:30, aliyyahlove
Most ionic compouds are crystalline solids at room temperature. true falseionic compounds are electrically neutral. true falseionic compounds generally have low melting points. true falsewhen melted, ionic compounds do not conduct electricity. true falsethe electrostatic attraction between an anion and a cation is an ionic bond. true false
Answers: 1

Chemistry, 23.06.2019 13:30, arianasg06
Explain the impact that changing the temperature has on a system in a state of dynamic equilibrium. what will happen when the temperature of an exothermic reaction mixture at equilibrium is increased?
Answers: 3
Do you know the correct answer?
1) How many kJ are absorbed when 45.2 g of water at 31.3 oC is heated to 76.9 oC? 2) Calculate the t...
Questions in other subjects:


Mathematics, 01.11.2019 23:31

Mathematics, 01.11.2019 23:31

Mathematics, 01.11.2019 23:31





History, 01.11.2019 23:31


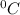 and Δθ is the change in temperature.
and Δθ is the change in temperature.




