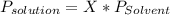Chemistry, 20.06.2020 05:57, kodiebclay
The vapor pressure of diethyl ether (ether) is 463.6 mm Hg at 25 °C. How many grams of aspirin , C9H8O4, a nonvolatile, nonelectrolyte (MW = 180.1 g/mol), must be added to 219.0 grams of diethyl ether to reduce the vapor pressure to 458.5 mm Hg ? diethyl ether = CH3CH2OCH2CH3 = 74.12 g/mol.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 20:00, jalenevoyles
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 23.06.2019 12:00, jluvit6135
In a reduction half-reaction, which amount is shown
Answers: 3
Do you know the correct answer?
The vapor pressure of diethyl ether (ether) is 463.6 mm Hg at 25 °C. How many grams of aspirin , C9H...
Questions in other subjects:


Mathematics, 28.08.2019 06:30


Mathematics, 28.08.2019 06:30


Mathematics, 28.08.2019 06:30

History, 28.08.2019 06:30