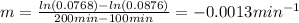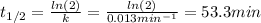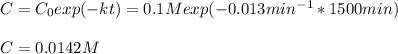3.The following data of decomposition reaction of thionyl chloride (SO2Cl2) were collected at a certain temperature and the concentration of SO2Cl2 were monitored as shown in the table.
SO2Cl2 (g) SO2 (g) + Cl2 (g)
Time (min)Conc. of SO2Cl2 (mol/L)
00.1000
1000.0876
2000.0768
3000.0673
4000.0590
5000.0517
6000.0453
7000.0397
8000.0348
9000.0305
10000.0267
11000.0234
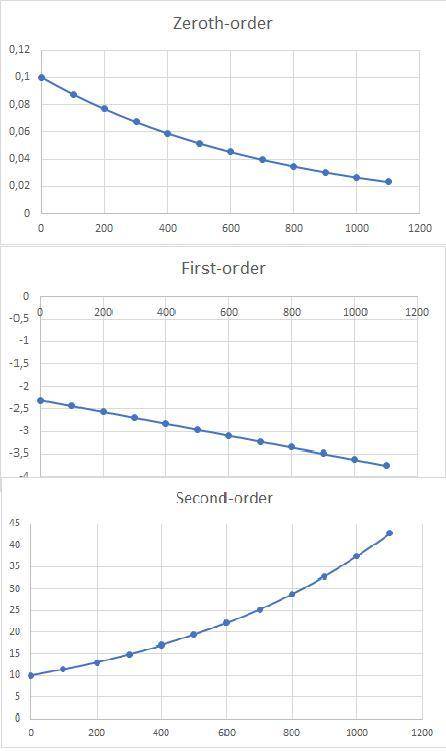
a)Determine graphically whether the kinetics of the reaction is zero order, first order or second order with respect to SO2Cl2 and then write the rate equation.
b)Determine the rate constant (k) of the reaction.
c)Determine the half-life (t½) for the reaction.
d)What will be the concentration of SO2Cl2 left in the reaction mixture at 1500 minutes?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
Do you know the correct answer?
3.The following data of decomposition reaction of thionyl chloride (SO2Cl2) were collected at a cert...
Questions in other subjects:


Chemistry, 12.11.2020 18:30

Mathematics, 12.11.2020 18:30






Biology, 12.11.2020 18:30