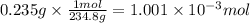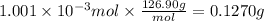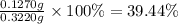Chemistry, 19.06.2020 03:57, smartboy2296
A sample of 0.3220 g of an ionic compound containing the iodide ion (I-) is dissolved in water and treated with an excess of AgHCO3. If the mass of the AgI precipitate that forms is 0.235 g, what is the percent by mass of I in the original compound? The molar mass of AgI is 234.8 g.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1
Do you know the correct answer?
A sample of 0.3220 g of an ionic compound containing the iodide ion (I-) is dissolved in water and t...
Questions in other subjects:



History, 10.12.2019 04:31

Geography, 10.12.2019 04:31

Mathematics, 10.12.2019 04:31




Arts, 10.12.2019 04:31