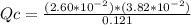Chemistry, 19.06.2020 03:57, ericchen4399
Consider the following reaction where Kc = 1.20×10-2 at 500 K. PCl5(g) PCl3(g) + Cl2(g) A reaction mixture was found to contain 0.121 moles of PCl5(g), 2.60×10-2 moles of PCl3(g), and 3.82×10-2 moles of Cl2(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals . The reaction A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Do you know the correct answer?
Consider the following reaction where Kc = 1.20×10-2 at 500 K. PCl5(g) PCl3(g) + Cl2(g) A reaction m...
Questions in other subjects:


Mathematics, 22.07.2021 01:00

English, 22.07.2021 01:00

English, 22.07.2021 01:00


Mathematics, 22.07.2021 01:00

Mathematics, 22.07.2021 01:00


English, 22.07.2021 01:00


![Qc=\frac{[C]^{c} *[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0689/6440/ada98.png)
![Qc=\frac{[PCl_{3}]*[Cl_{2} ]}{[PCl_{5} ] }](/tpl/images/0689/6440/81d8b.png)
![[PCl_{3} ]=\frac{2.60*10^{-2} moles}{1 liter} =2.60*10^{-2} \frac{moles}{L}](/tpl/images/0689/6440/fd446.png)
![[Cl_{2} ]=\frac{3.82*10^{-2} moles}{1 liter} =3.82*10^{-2} \frac{moles}{L}](/tpl/images/0689/6440/fced6.png)
![[PCl_{5} ]=\frac{0.121 moles}{1 liter} =0.121 \frac{moles}{L}](/tpl/images/0689/6440/529d8.png)