
Chemistry, 19.06.2020 03:57, supergraciepie
A titanium cube contains 3.10•10^23 atoms. The density of a titanium is 4.50g/cm^3. What is the edge length of the cube? PLEASE HELPPP! :(
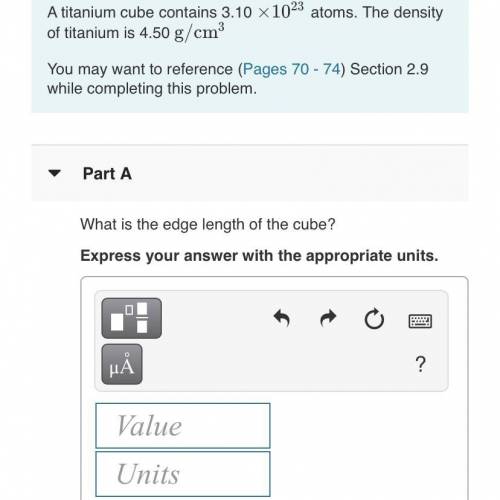

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 15:30, tristen2001
Select the correct answer. the gas in a sealed container has an absolute pressure of 125.4 kilopascals. if the air around the container is at a pressure of 99.8 kilopascals, what is thegauge pressure inside the container?
Answers: 3

Chemistry, 23.06.2019 19:40, texas101st78
Alab technician needs to create 570.0 milliliters of a 2.00 m solution of magnesium chloride (mgcl2). to make this solution, how many grams of magnesium chloride does the technician need? refer to the periodic table for . express your answer to three significant figures.
Answers: 3
Do you know the correct answer?
A titanium cube contains 3.10•10^23 atoms. The density of a titanium is 4.50g/cm^3. What is the edge...
Questions in other subjects:


History, 11.04.2021 04:20



French, 11.04.2021 04:20



Mathematics, 11.04.2021 04:20

Mathematics, 11.04.2021 04:20






