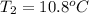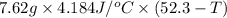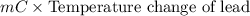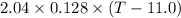Chemistry, 19.06.2020 00:57, straightbarz5916
A 2.04 g lead weight, initially at 10.8 oC, is submerged in 7.62 g of water at 52.3 oC in an insulated container. clear = 0.128 J/g oF; water = 4.18 J/goC. What is the final temperature of both the weight and the water at thermal equilibrium

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3


Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
Do you know the correct answer?
A 2.04 g lead weight, initially at 10.8 oC, is submerged in 7.62 g of water at 52.3 oC in an insulat...
Questions in other subjects:

Mathematics, 25.10.2021 14:00



Biology, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00



Mathematics, 25.10.2021 14:00



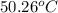 .
.