
Consider the following reaction where Kc = 0.159 at 723 K. N2(g) + 3H2(g) 2NH3(g) A reaction mixture was found to contain 1.97×10-2 moles of N2(g), 3.82×10-2 moles of H2(g) and 5.27×10-4 moles of NH3(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals 9.63x10^4 . The reaction b A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 23.06.2019 13:30, jharrington583
Which of the following is true regarding chemical and nuclear reactions?
Answers: 1
Do you know the correct answer?
Consider the following reaction where Kc = 0.159 at 723 K. N2(g) + 3H2(g) 2NH3(g) A reaction mixture...
Questions in other subjects:


Biology, 17.10.2019 05:10


Geography, 17.10.2019 05:10

History, 17.10.2019 05:10

History, 17.10.2019 05:10



Mathematics, 17.10.2019 05:10

Biology, 17.10.2019 05:10


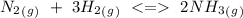
![K_e_q=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0689/0595/b557b.png)
![Q_c=\frac{[5.27X10^-^4]^2}{[1.97X10^-^2][3.82X10^-^2]^3}=0.251](/tpl/images/0689/0595/172a3.png)




