
In the first step of glycolysis, the given two reactions are coupled. reaction 1:reaction 2:glucose+Pi⟶glucose-6-phosphate+H2 OATP+H2O⟶ADP+PiΔG=+13.8 kJ/molΔG=−30.5 kJ/mol reaction 1:glucose+Pi⟶glucose-6-phosphate+H2 OΔG=+13.8 kJ/molreaction 2:ATP+H2O⟶ADP+PiΔG=−30.5 kJ/mol Answer the four questions about the first step of glycolysis. Is reaction 2 spontaneous or nonspontaneous?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:10, lilque6112
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2
Do you know the correct answer?
In the first step of glycolysis, the given two reactions are coupled. reaction 1:reaction 2:glucose+...
Questions in other subjects:




English, 08.01.2021 05:20



Chemistry, 08.01.2021 05:20

Social Studies, 08.01.2021 05:20


French, 08.01.2021 05:20


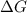 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous
 glucose-6-phosphate + H₂O, ΔG = +13.8 kJ/mol
glucose-6-phosphate + H₂O, ΔG = +13.8 kJ/mol



