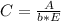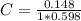Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, misspicafunpoke
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2



Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
Do you know the correct answer?
After creating a Beer's Law plot using standard solutions of Q, you determined the slope of Beer's L...
Questions in other subjects:





Mathematics, 26.05.2021 04:00



Spanish, 26.05.2021 04:00



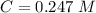
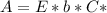 . On this equation we will have:
. On this equation we will have: