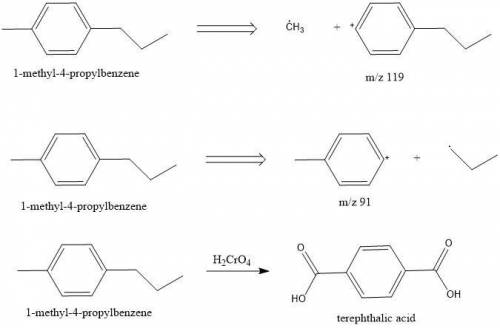
Compounds A and B (both C10H14) show prominent peaks in their mass spectrum at m/z 134 and 119. Compound B also shows a less prominent peak at m/z 91. On vigorous oxidation with chromic acid, compound A is nonreactive while compound B yielded terephthalic acid.
From this information, deduce the structures of both compounds, and then draw the structure of B.
You do not have to consider stereochemistry
You do not have to explicitly draw H atoms

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2



Chemistry, 23.06.2019 01:00, Angelofpink1143
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
Do you know the correct answer?
Compounds A and B (both C10H14) show prominent peaks in their mass spectrum at m/z 134 and 119. Comp...
Questions in other subjects:


Mathematics, 08.05.2021 05:30


Mathematics, 08.05.2021 05:30

Mathematics, 08.05.2021 05:30


Arts, 08.05.2021 05:30

Chemistry, 08.05.2021 05:30

Mathematics, 08.05.2021 05:30

Mathematics, 08.05.2021 05:30