Chemistry, 14.06.2020 14:57, kenisonpaigebosma
Chlorine dioxide is used as a disinfectant and bleaching agent. In water, it reacts to form chloric acid (HClO3),
according to the following balanced equation:
6 ClO2 + 3 H2O → 5 HClO3 + HCl
If excess ClO2 is mixed with 18.0 mL of H2O (d = 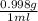 ) how many grams of HClO3 are formed?
) how many grams of HClO3 are formed?
PLEASE DO NOT JUST GIVE THE ANSWER!!! I really would like to learn HOW to do this so please give explanations for each step as you solve it. Thanks!

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
Do you know the correct answer?
Chlorine dioxide is used as a disinfectant and bleaching agent. In water, it reacts to form chloric...
Questions in other subjects:

Mathematics, 14.04.2020 16:49

Biology, 14.04.2020 16:49


Mathematics, 14.04.2020 16:50

Mathematics, 14.04.2020 16:50



Health, 14.04.2020 16:50