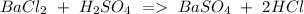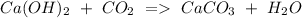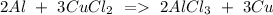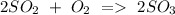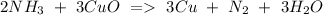Balance the following chemical equations.
a) Ba Cl2 + H2SO4 BaSO4 + HCl.
b) Calcium hy...

Chemistry, 13.06.2020 11:57, flyingcerberus1408
Balance the following chemical equations.
a) Ba Cl2 + H2SO4 BaSO4 + HCl.
b) Calcium hydroxide + Carbon dioxide Calcium carbonate + Water.
c) Aluminum + Copper chloride Copper + Aluminum chloride
d) Sulphur dioxide + Oxygen Sulphur trioxide
e) NH3+ CuO Cu + N2 + H2O

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 01:20, hflores0001
How can parts of a solution be separated by chromatography?
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Biology, 31.05.2021 23:00





English, 31.05.2021 23:00

English, 31.05.2021 23:00

Mathematics, 31.05.2021 23:00


Business, 31.05.2021 23:00