
Hydrogen bromide decomposes when heated to 437C according to the equation: 2HBr(g) H2(g) Br2(g). If the reaction starts with 2.15 mol of hydrogen bromide in 1.0 liter, and decomposes to 36.7%, what is the equilibrium constant of the decomposition of hydrogen bromide

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 23.06.2019 00:00, bryn2433
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
Do you know the correct answer?
Hydrogen bromide decomposes when heated to 437C according to the equation: 2HBr(g) H2(g) Br2(g). If...
Questions in other subjects:

Health, 14.06.2021 21:20




Mathematics, 14.06.2021 21:20

Mathematics, 14.06.2021 21:20

Mathematics, 14.06.2021 21:20


Mathematics, 14.06.2021 21:20


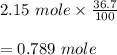
![K_c=\frac{[H_2][Br_2]}{[HBr]^2} \\\\=\frac{(0.395)(0.395)}{(1.361)^2} \\\\=\frac{0.156025}{1.852321} \\\\=0.084](/tpl/images/0685/5664/e5d29.png)




