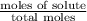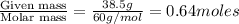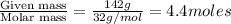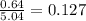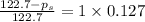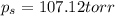Chemistry, 13.06.2020 23:57, reginaldlegette
Calculate the vapor pressure (in torr) at 298 K in a solution prepared by dissolving 38.5 g of the non-volatile non-electrolye urea {CO(NH2)2} in 142 g of methanol. The vapor pressure of methanol at 298 K is 122.7 torr. Give your answer to 2 decimal places.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, akeemedwards12
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 07:50, carlosiscr7
Many reactions take place in aqueous solution. when potential reactants are mixed, a reaction will occur if there is some driving force that favors the formation of products. it is often convenient to categorize reactions in terms of these driving forces: precipitate formation, in which an insoluble solid is formed, weak electrolyte formation, as in a neutralization reaction involving water, or transfer of electrons, as in a redox reaction. these reactions can be represented by full molecular equations, which contain all species in the reaction mixture, or by net ionic equations, which show only the species that actually undergo a change. the latter does not contain the spectator ions, which do not undergo a net change or do not take part in the reaction. part a when the following two solutions are mixed: k2co3(aq)+fe(no3)3(aq) the mixture contains the ions listed below. sort these species into spectator ions and ions that react. drag the appropriate items to their respective bins. view available hint(s) spectator ions ions that react part b what is the correct net ionic equation, including all coefficients, charges, and phases, for the following set of reactants? assume that the contribution of protons from h2so4 is near 100 %.ba(oh)2(aq)+h2so4(aq)→ express your answer as a chemical equation. view available hint(s) nothing provide feedback
Answers: 3


Chemistry, 23.06.2019 05:30, brianrodriguez2005
What is the body’s main processing system? it uses input from various parts to control voluntary and involutiontary movement. it’s composed of two main parts-the brain and spinal cord. a. nbs b. cns c. ans d. pns
Answers: 1
Do you know the correct answer?
Calculate the vapor pressure (in torr) at 298 K in a solution prepared by dissolving 38.5 g of the n...
Questions in other subjects:


Mathematics, 18.11.2020 23:00


Mathematics, 18.11.2020 23:00

Mathematics, 18.11.2020 23:00

Health, 18.11.2020 23:00

History, 18.11.2020 23:00

Mathematics, 18.11.2020 23:00


Mathematics, 18.11.2020 23:00


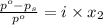
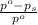 = relative lowering in vapor pressure
= relative lowering in vapor pressure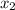 = mole fraction of solute =
= mole fraction of solute =