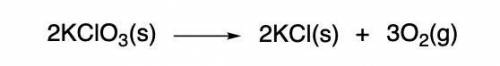
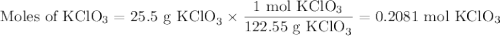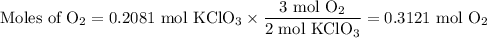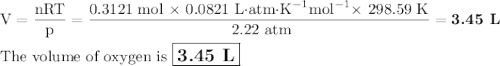I NEED HELP, THANKS! Using the Ideal Gas Law, PV = nRT, where R = 0.0821 L atm/mol K, calculate the volume in liters of oxygen produced by the catalytic decomposition of 25.5 g potassium chlorate according to the following reaction. The oxygen is collected at 2.22 atm and 25.44°C. Express your answer to the correct number of significant figures.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, elizediax8683
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
Do you know the correct answer?
I NEED HELP, THANKS!
Using the Ideal Gas Law, PV = nRT, where R = 0.0821 L atm/mol K, calculate the...
Questions in other subjects:


English, 05.12.2021 21:50

History, 05.12.2021 21:50



English, 05.12.2021 21:50



Mathematics, 05.12.2021 21:50

History, 05.12.2021 21:50